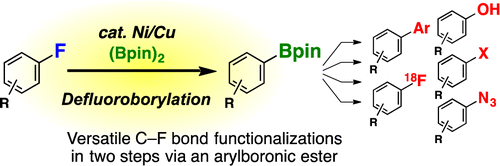
Ni/Cu-Catalyzed Defluoroborylation of Fluoroarenes for Diverse C–F Bond Functionalizations
Using a mixed catalytic system to promote the transformation of aromatic fluorides into boronate partners suitable for further functionalization.
This is another fine example of current organometallic chemistry. More than 20 years ago, while studying in the University, they told us that being the C-F bond one of the strongest in nature, breaking it was impossible in most conditions and to do organometallic couplings with boronates/boronics you needed a iodine, bromine or triflate. Mostly. Happily, this is no longer true. And in this paper by Niwa and Hosoya (RIKEN Center, Kobe, Japan), you will see how far our knowledge in organometallic couplings has gone.
Apparently, the authors selected aromatic fluorides because the chemical stability of C-F bonds, so you can avoid less stable substrates. And after some thinking and a lot of experiments (as usual), they came up with an efficient nickel and copper cocatalytic system which can transform the selected substrates into boronates. The conditions for the transformation are heating a mixture of the substrate with bis(pinacolato)diboron, Ni(Cod)2, PCy3, CuI and CsF in toluene at 80 °C. The results are remarkable, with conversions ranging from 50 to 100%. For some substrates, a threefold increase in the amounts of catalysts and base were required, but still the reaction does not work for substrates with strong electrowithdrawing groups.
Finally, the authors use the new conditions to prepare a boronate derivative of Dihydrofluvastatin, which in turn is derivatized in a number of other products. As criticism, a) GC is not a suitable technique to give yields, but conversions and b) a broader range of substrates would be welcomed (no substrates with substituents in ortho or meta; no halogens. Ah, life.)
J. Am. Chem. Soc. 2015, 137(45), pp 14313–14318.
See: 10.1021/jacs.5b10119