Synthesis of Heteroaryl Sulfonamides from Organozinc Reagents and 2,4,6-Trichlorophenyl Chlorosulfate
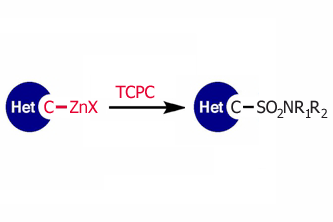
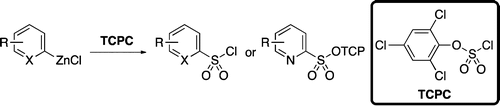
Access to sulfonated heterocycles using zincates and the reagent TCPC.
Access to sulfonated heterocycles using zincates and the reagent TCPC.
It is not the first time that I talk on this blog about some gaps in the synthetic methods available to prepare small, simple building blocks. Some chemists I have talked to think that ‘now we can prepare everything’. Maybe this is true, but if you need handling toxic or hazardous reagents in the lab, or doing five synthetic steps just to prepare a building block, well, then I think there is plenty of room for improvement.
And preparation of heteroaryl sulfonamides is one of those spots. The authors of the paper, Buchwald et al. (MIT, MA, USA), have already done some contributions on this topic, but further improvements were needed. And here we are. The new protocol makes use of the reagent 2,4,6-Trichlorophenyl Chlorosulfate (TCPC), which was first described in the literature in the sixties, and whose preparation is included in the supporting info. This reagent is key by several reasons, maybe the most important because on reaction with heteroaryl zinc reagents it yields stable, solid sulfonate esters of trichlorophenol (TCP), wich in turn can be used to prepare amides by reaction with the corresponding amines.
So to summarize: prepare TCPC, and react it with the heteroaryl zinc bromide or chloride in THF at 0 °C for a couple of hours. Isolate the TCP ester and react it with the amine you need, from aqueous NH3 to morpholine, aniline or others. The paper contains examples with pyridine 2-sulfonamides (several examples), pyrazoles, thiophenes, indoles, pyrroles, benzofuranes and benzothiophenes. However, I would preferred very much less variety on the heterocycles, and more info about the potential interactions with the heterocycles substituents. Anyway, a good addition to our weaponry.
Org. Lett. 2015, Article ASAP.
See: 10.1021/acs.orglett.5b01540