Practical Radical Cyclizations with Arylboronic Acids and Trifluoroborates
A practical radical cyclization using boronic acids and trifluoroborates.
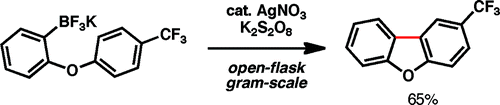
Hmmm, this came like a shock. I was going through the ASAP and after seeing this I thought ‘hey, they have forgotten the Pd somewhere’. But no, the Palladium is not needed. Baran et al. (The Scripps Research Institute, CA, USA) describe a method to carry out cyclizations using boronic acids and trifluroborates, but NO Palladium! In fact, the method is a radical cyclization, triggered by the use of catalytic AgNO3 and stoichiometric potassium persulfate. For people like me, coming from an age where ‘practical radical cyclizations’ involved tributyltin hydride and AIBN in acetonitrile, this method looks wonderful. They report more than 20 examples, preparing a wide range of tri- and bicyclic scaffolds. More important, the method can be carried out in a multigram scale, with cheap reagents, on an open vessel, with a mixture of water and trifluoromethylbenzene (this is exotic, yes). So the next time you need a scaffold, take a look at this paper.
Org. Lett., Article ASAP. See: 10.1021/ol2023505