From Carboxylic Acids to the Trifluoromethyl Group Using BrF3
A method to convert carboxylic acids into trifluoromethyl groups.
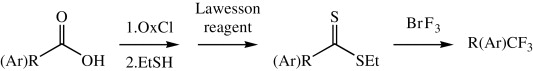
This paper by Rozen et al. (Tel-Aviv University, Israel) shows a new method for transforming a carboxylic acid into a CF3 group, a useful bioisostere. Typical methods for introducing this moiety include the Prakash–Rupert’s reagent (CF3SiMe3), fluoroform (CF3H), trifluoromethyl halides (i.e., CF3I), and electrophilic trifluoromethylations. Carboxylic acids can be turned into CF3 using sulfur tetrafluoride (SF4).
This new protocol uses also carboxylic acids as starting materials, which are first transformed into dithioesters by sequential reaction of a starting acid chloride with ethanethiol, followed by the use of Lawesson’s reagent. The dithioester is then dissolved in CFCl3, cooled to 0 °C, and further reacted with BrF3 (commercially available) for only 2–3 min. Yields are usually very good (around 70%) and the method can be applied both to aryl and alkylcarboxylic acids. No data are given on the use of heteroarylcarboxylic acids. The method can be long and smelly, but compared to other methods we have used for the introduction of CF3… it looks wonderful!
Tetrahedron 2010, 66(20), pp 3579–3582. See: 10.1016/j.tet.2010.03.045