Cross-Coupling of Cyclopropyl- and Cyclobutyltrifluoroborates with Aryl and Heteroaryl Chlorides
The introduction of those small funny cycloalkyls is becoming easier.
When people receive the first notions about organic chemistry, usually they are amused by that way of drawing chemical structures: you draw small pentagons, hexagons, triangles and squares. It is like a child’s play. Then you grow up and receive your first serious notions about organic chemistry, and learn that those small triangles and squares are hard to get. The development of mild methods for the incorporation of cyclopropyl and cyclobutyl group into complex molecules has become increasingly important in the last years, since these groups have interesting properties for biological receptors. The Suzuki-Miyaura reaction, being mild and flexible, has emerged as an attractive method for the introduction of these groups, but up to now most of the work has centered in the use of cycloalkylboronic acids, which have several problems of stability and cost. Moreover, most of the work has been done on the usual substrates, bromides and iodides.
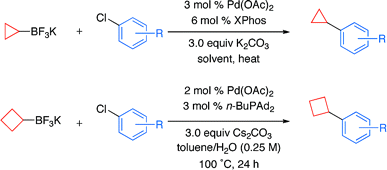
We present here a paper of Molander (Philadelphia, PA, USA) that addresses that problems as continuation of his efforts on alkyltrifluoroborates. They have developed conditions for the coupling of potassium cyclopropyl- and cyclobutyltrifluoroborate with aryl and heteroaryl chlorides which allow the mild and effective introduction of those moieties. Typical reaction conditions involve the addition of the aryl chloride, potassium cycloalkyltrifluoroborate, Pd(OAc)2, XPhos, K2CO3 in CPME/H2O at 100 °C for 24 h. When heteroaryl chlorides are used the ligand is changed to n-BuPAd2 and the base to Cs2CO3. Though the aryl chlorides selected are just representative (some more ortho- substituted compounds and wider diversity would be welcome), the heteroaryl chlorides are well represented, including pyridines, quinolines, quinoxalines, thiophenes and furanes. Four examples of the cyclobutyl introduction are also included. In all cases yields range from very good to excellent.
J. Org. Chem., 2008, 73, pp 7481–7485. See: 10.1021/jo801269m