Copper-Catalyzed Selective Arylations of Benzoxazoles with Aryl Iodides
Still trying to decide if this is a joke or not. Any help is welcomed.
To keep abreast of the latest developments in some ACS publications (JOC, JACS, OL, JMC and others), I use a free app for iPad called ACS Mobile . This application is really friendly: you just set up the journals you want to follow, and it lists for you the last ACS ASAP papers, displaying the title, authors, and the graphic abstract. If you touch the paper it will open another view with more details, including the written abstract. You can do other things, but for me, it’s papers, and the graphic abstract is quite useful, because it allows me to get a grasp of the paper’s content with a quick look.
Or it should be. Really, one of these days I will write another entry about the graphic abstracts. But not today. Today this is about one paper that took my attention due exactly to the graphic abstract. See below.
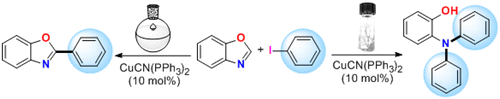
I spent 10 seconds or so trying to get it. Same conditions, two completely different outcomes of the reaction? Uh? And then I noticed the graphic: flask or vial. And the understanding (of the image, that is), came reading the abstract: “The selectivity between ring-opening N-arylation and C-arylation was controlled by the choice of reaction vessel.” Are you kidding me? This I must read. And so I did. And I am still scratching my head, because I do not know if this a joke, or what. The authors do not give much info about that vessel selection, but this:
[…] While the C-2 arylated product (4aa) was the major product using Miura’s condition,4a changing the reaction vessel from a round-bottomed flask to a screw-capped reaction vial shifts the preference from an arylation of the C-2 position to a ring-opening N-arylation.[…]
Not much information, uh? But the icing on the cake is this last comment:
Finally, perfect selectivity of arylation was revealed by the simple choice of reaction vessel. In the optimized condition (entry 9 in Table 1), the reaction was performed in a screwcapped vial, and formed the ring-opened double-arylated product 3aa, as the sole product. However, by changing the reaction vessel from a closed, and assuming pressured, screwcapped vial to an open round-bottomed flask, the C-arylated product 4aa was the major product and isolated in high yields (85%, entry 10).
And that is the last comment they do about this incredible, simple and cheap solution to the selectivity problem. Of course, so simple that is of pure genius! I have checked with half a dozen chemists and their reactions were more or less similar, but unfortunately I cannot reproduce them here. The authors must be experiencing the strong effect of some unchecked variable (stirring, presence of oxygen, pressure?), which is not unusual. We had once a synthesis where the Project Manager claimed that the reaction yield was strongly dependent on the vessel’s neck, but she inmediately said that it was clearly a case of stirring messing up the reaction, and then she demonstrated it by reproducing the reaction succesfully in the Radley’s tubes, and later running the reaction in parallel to get grams of the stuff while avoiding the difficult scale up. But not here. They have not a problem with the yield, but a completely different reaction mechanism just by choosing the proper vessel. And it seems that they are not worried or surprised about it, at least not enough to go into further details or provide some rational explanation.
What I find more disturbing is not that this piece of… science made its way to the Journal of Organic Chemistry, not less, but the fact that apparently did it so with the approval of several referees. In the acknowledgments of the paper, they thank a couple of people “for helpful discussions”. Honestly, I cannot believe that nobody asked questions about the vessel issue.
Well, maybe this is an April’s Fool Day joke which came a bit earlier than expected… or not. But honestly, is this what they publish in the Journal of Organic Chemistry these days?
J. Org. Chem. 2015, ASAP.
See: 10.1021/acs.joc.5b00019