In the last forty years, metal-catalyzed chemical reactions that create new carbon–carbon bonds have become an essential tool for chemists. Other classical reactions, such as aldolic condensation, have become less and less important, while the scope and types of these organometallic couplings have expanded greatly. Reactions that were at first good only for Csp2–Csp2 bond generation, now cover the formation of any carbon–carbon bond. Moreover, the development of other organometallic couplings has addressed the problem of creating C–X bonds using as partners amines, sulfides, and alcohols. This increasing importance of organometallic couplings was recognized in 2010 with the Nobel prize in Chemistry for Richard F. Heck, Ei-ichi Negishi and Akira Suzuki.
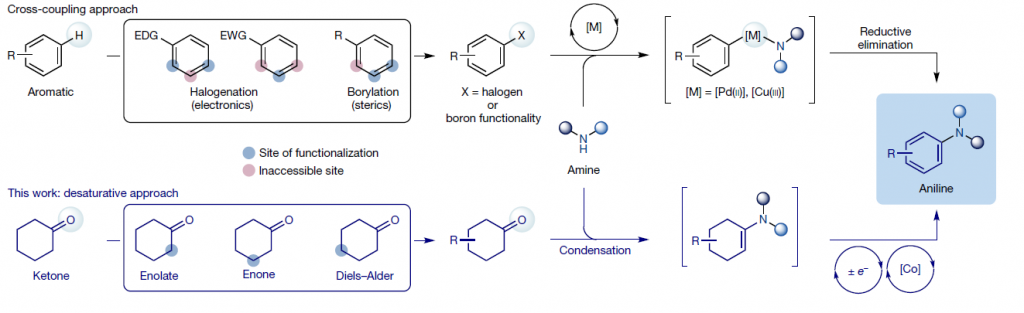
The formation of C–N bonds is one of those reactions that has gone a long way since the seminal works by Ullmann using Cu catalysis. In the last two decades, the development of the Buchwald–Hartwig protocol allowed the preparation of (hetero)aromatic amines by coupling a wide range of amines with (hetero)aryl partners including halides (Cl, Br and I), activated phenols (tosylates, mesylates), and many more. The Chan–Lam reaction is another alternative, exploiting also Cu catalysis with amines and (hetero)arylboronic acids as coupling partners. It can also be used to generate C–O and C–S bonds. So, if you add to those three couplings the humble reductive amination, where substituted (hetero)arylamines can be generated using (hetero)arylanilines and ketones, it seems that medicinal chemists have a wide range of options available. The issue, when you think about it, arises because the (hetero)aromatic ring in all those reactions is already fixed, although finding the correct partner for the coupling can be challenging due to the reactivity of the products or accessibility to the correct substitution partner.
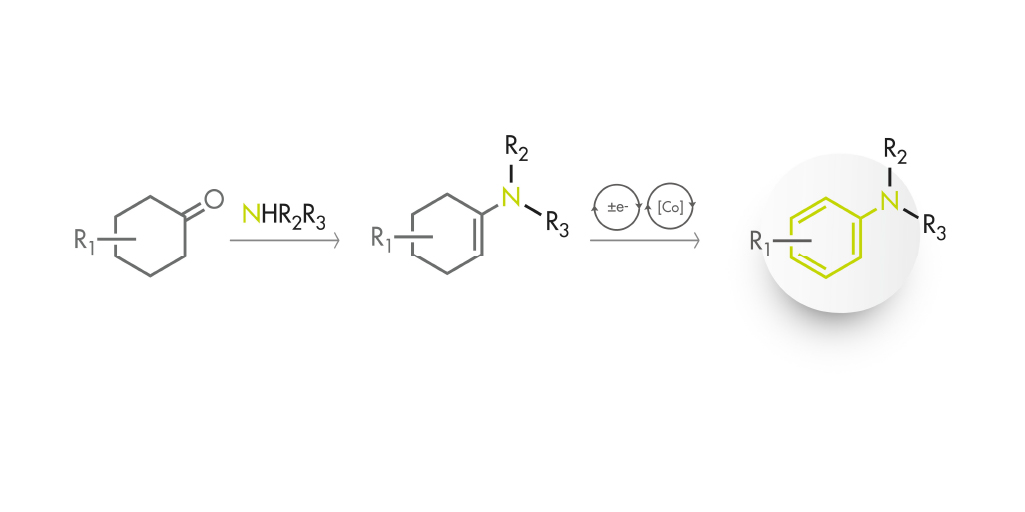
So the question is, what if we could generate the aromatic ring in situ instead of taking it as a given? This is the starting point for the work by Leonori et al. (University of Manchester, UK). They have made use of the latest advances in photocatalysis to develop a photoredox-cobalt dehydrogenative coupling that makes use of classical and state-of-the-art chemistry to prepare (hetero)anilines from amines and ketones.
The synthesis starts with a suitably substituted secondary amine (some of which are notoriously difficult to couple under Buchwald–Hartwig conditions…) and a cyclohexanone. The initial condensation yields an enamine, which through a Single Electron Transfer (SET) mechanism transfers an electron to an excited Ir(III) complex. The enaminium radical so generated loses H+ to generate a neutral beta-enamine radical. Here enters the co-catalyst, a Co(II) complex, which is responsible for the oxidation of the beta-enamine to a dienamine. A second SET/oxidation cycle yields finally a product with an aromatic ring fabricated from the cyclohexanone ring.
We will not go into the details of the mechanism (you can read the full article) but it is enough to say that the reaction has a neat equation: amine plus ketone in the presence of [Ir(dtbbpy)(ppy)2]PF6 (2 mol%), cobaloxime (4 mol%), AcOH (20 mol%), DABCO (1.5 equiv.) in CH3CN at 30–40 ºC for 24 h to yield the amine plus water and hydrogen. Worry not, because the Ir and Co catalysts are commercially available, and the hardware is more or less that used in a bunch of other photoredox reactions.
The authors explored next the scope of the amine, which can be pretty much anything: dialkylamines, primary amines, hindered primary amines (some of which would look daunting for use in other couplings), (hetero)anilines… and the reaction works with very good results most of the time. The same happens when they explore the scope of the ketone. Ironically, they found that there are not many ketones with the required pattern available, so they resorted to old fashion chemistry (enolates, conjugate additions, Diels–Alder cycloadditions…) to prepare the substrates needed for such a state-of-the-art method like this. And it works with all sorts of cyclohexanones, tetralones and fused heteroketones. What about introducing a simple nitrogen atom, that is, synthesizing an aniline? Just switch to NH3(7N in MeOH) and once again, they have good results using substrates like terpenes and sexual hormones.
So here we are, a new method for the synthesis of (hetero)anilines using old fashion chemistry and advanced photocatalysis.
A photochemical dehydrogenative strategy for aniline synthesis, Nature, 2020, 584, pp. 75–81. See: 10.1038/s41586-020-2539-7