Palladium-Catalyzed, Direct Boronic Acid Synthesis from Aryl Chlorides: A Simplified Route to Diverse Boronate Ester Derivatives
Advances in the preparation of boron coupling partners.
In the Top Five of our last issue we have included a paper on the preparation of benzylic boronates by using a catalytic amount of magnesium. Now we present the last improvements on this field by the hand of Molander.
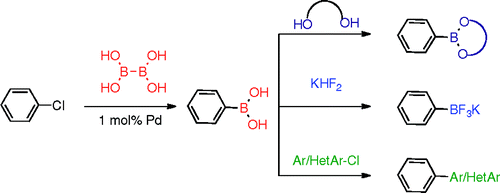
The group of Molander has worked in the preparation of boronic acids using Palladium catalysis, but a source of boron cheaper than bis(pinacolato)diboron would be much desirable. And they have found that using B2(OH)4, tetrahydroxydiboron, works fine. The trick is, as usual, using the correct Palladium catalyst. In this case it is the same used by Buchwald in another paper of our Top Five, but combining the catalyst with X-Phos. In a typical experiment, the aryl halide, 1 mol% of the precatalyst, X-Phos (2 mol%), NaOt-Bu (1 mol%), KOAc (3 equiv) and B2(OH)4 (1.5 equiv) in EtOH at 80 °C for 18 h yields and intermediate boronate (derived from the EtOH used as solvent) that can be further transformed by adding any of the stabilizing reagents currently in use: pinacol, 2,2-dimethyl-1,3-propanodiol, MIDA, and others. Using hexane renders the boronic also, and adding KHF2 the trifluoroborate. If the costs in catalyst, ligand and reagents are favourable to the current methods, maybe we are seeing a revolution in boron partners. Have I failed to tell you that the starting aryl halide is a chloride? Well, it is. And to round it, Molander presents also a one-pot Suzuki method using the boronate solution, which can be coupled with another aryl or heteroaryl chloride. Impressive… very impressive.
J. Am. Chem. Soc., 2010, 132 (50), pp 17701–17703. See: 10.1021/ja1089759