Pd-Catalyzed Direct Arylation of Tautomerizable Heterocycles with Aryl Boronic Acids via C–OH Bond Activation Using Phosphonium Salts
If this method can be further expanded, the days of phosphorus oxychloride are over. Keep reading.
Some reagents are sadly familiar for medicinal chemists working with heterocycles. POCl3 is probably one of these reagents. It is the almost universal source of Cl when looking for a way to change a cyclic amide into a group amenable to coupling; unlike aryl chlorides, heteroaryl chlorides can be easily coupled. When the use of POCl3 results troublesome, we look for a triflate, resorting to Tf2O or TfPh2N, but all these reagents are toxic, air and/or moisture unstable, and user unfriendly.
Very recently, PyBOP and PyBroP were presented as alternative reagents for this functionalization, which were found to work very well in direct C–N, C–O, C–S, and C–C bond formations. The work now presented by Kang (Johnson & Johnson, USA) is a follow-up of the use of these reagents. Instead of using a phosphonium intermediate to introduce amines, etc. through nucleophilic substitution, they have modified the protocol to carry out a Suzuki reaction.
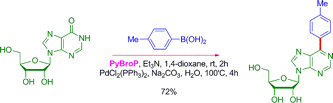
The results presented in the paper include ten examples of coupling between different pyridones, pyrimidones, quinoxalinones, among others, and selected boronic acids. The yields go from very good to excellent, even with hindered boronic acids, improving the coupling results of aryl phosphonates. As a final example of the method versatility, a purine nucleoside is coupled using PyBroP and Et3N in 1,4-dioxane at r.t. for 2 h and adding then p-tolylboronic acid, Na2CO3, PdCl2(PPh3)2, and water. Heating the mixture at 100 °C during 4 h gives the product in a 72% yield: no protection-activation-coupling-deprotection sequence at all, just a straightforward activation-coupling.
I suppose the next logical step is trying other couplings. If they work, the days of POCl3 in medicinal chemistry are over.
J. Am. Chem. Soc., 2008, 130 (34), pp. 11300–11302. See: 10.1021/ja804804p