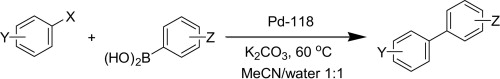
Development of a general Suzuki reaction protocol
This is one of the best papers I have seen lately. The authors (former Pfizerites, as far as I remember) describe a general protocol for the Suzuki reaction (yes, another one), using Pd-118 (Johnson Matthey’s name for Pd(dbpf)Cl2, 1,10-bis(di-tert-butylphosphino)ferrocene palladium dichloride). The protocol works nicely with aryl bromides and chlorides, even hindered ones, with low loadings of catalyst (1-2%). The protocol has been developed with an industrial use in mind, so many people around, not only medicinal chemists (yes, the protocol works also with heterocycles), will find it very useful.
Additionally, I find really interesting that this people, with a non-academic background, can give lessons to many people around on how to design a methodology study. And they are not afraid of using substrates with ortho-substituents, not to say that some substrates do not work.
Tetrahedron 2012, 68(30), pp 6010–6017.
See: 10.1016/j.tet.2012.05.030