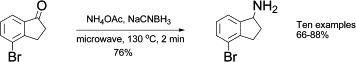Microwave-accelerated reductive amination between ketones and ammonium acetate
Reductive amination under microwave irradiation conditions.
Reductive Amination is probably one of the most used reactions in medicinal chemistry and the method of choice for preparing substituted amines. The reaction has a few shortcomings and limitations. One is related with the type of amines you can prepare. It is wonderful for secondary and tertiary amines, but preparing primary amines can be sometimes quite challenging.
Faced up with the need to prepare these type of amines, the chemist from Lexicon Pharmaceuticals (Germany) found the usual problems and turned to microwave synthesis as a way to accelerate things. After some experimentation they found out that indeed the use microwaves is a great improvement compared with the thermal reaction: Shorter reaction time, better yield and more important, no dimeric product resulting from the tandem addition of two molecules of ketone to the nitrogen atom is formed.
The final protocol requires NH4OAc (15 eq.) and NaCNBH3 (120 mol%) to be added to a solution of the ketone in EtOH. The mixture is heated under microwave irradiation at 130 °C for 2 min. Curiously, no details on the power used are given. Yields are usually higher than 80%. Interestingly, they found out that the use of another common reduction agent, Na(OAc)3BH, gives predominantly the dimeric product under these conditions.
Tetrahedron Letters 2010, 51 (39), pp 5210-5212. See: 10.1016/j.tetlet.2010.07.156