Copper-Mediated Direct Arylation of Benzoazoles with Aryl Iodides
From the Far East, here comes another interesting paper about C–H functionalization, in this case on benzoazoles.
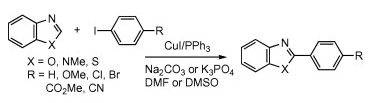
As we have stated on another paper selected for this issue of our newsletter, direct C–H functionalization is very attractive. In this context, it has been known for some time that azoles can be directly coupled with aryl halides, typically using palladium or rhodium. In a recent paper, Miura et al. (Osaka, Japan) have demonstrated that the same coupling can be effectively carried out using a catalytic system with CuI, PPh3, and a base (Na2CO3 or K3PO4) in DMF. The protocol has been applied to benzoxazole, benzimidazole, and benzothiazole, with no substitution in the ring, and the conditions described are thermal, but this new method may be used to quickly expand the diversity in a chemical series of substituted benzoazoles.
Tetrahedron Lett. 2008, 49, pp. 1598–1600. See: 10.1016/j.tetlet.2008.01.042