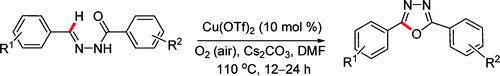Cu(II) Catalyzed Imine C–H Functionalization Leading to Synthesis of 2,5-Substituted 1,3,4-Oxadiazoles
Here you have a simple method to prepare 2,5-substituted 1,3,4-oxadiazoles: take an aromatic aldehyde, take a benzahydrazide, run a condensation, and with the resulting N-benzylidinebenzahydrazide run a reaction using Cu(OTf)2 as catalyst; you will obtain the cyclized product. Many substituents can stand the reaction conditions (though as usual it seems the authors prefer substituents in para…), a nice trend is stablished, and you can even put some heterocycles here and there. The paper gives a couple of interesting hints on how extend the scope of the procedure to other systems different than diaryl, but we will leave this as an exercise for the reader.
Org. Lett., 2011, 13 (22), pp 5976–5979. See: 10.1021/ol202409r