Rational Ligand Design for the Arylation of Hindered Primary Amines Guided by Reaction Progress Kinetic Analysis
A working protocol for the Buchwald-Hartwig amination with hindered primary amines.
When I finished my PhD, many years ago (honestly, I do not want to say how many), the Palladium catalyzed amination was still a reaction under development. Some initial protocols have been published, but the reaction was far from being a standard method to prepare amines. Only after a more general protocol was developed the reaction became a common tool, one that you could consider when designing a new route. But even after so many years the reaction has its limits, and one of the most important is the application to bulky amines. When you had one of those cases in your hands (and probably most medicinal chemists have already found themselves in this predicament), you searched the literature looking for some special conditions that could do the trick. For example, this user’s guide by Surry and Buchwald.
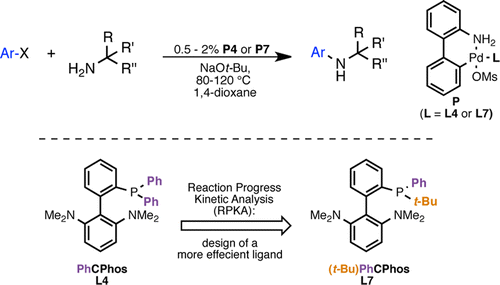
Buchwald himself (MIT, Mass., USA), has published a new work dealing with the coupling of hindered primary amines. The trick is obviously the use of a new catalyst, not very different from the known systems but containing a new ligand, a biaryl(alkyl)arylphosphine (see the scheme below). This new conditions can be applied to (hetero)aryl chlorides and bromides to give the bulky coupling products in excellent yields. Another step forward.
J. Am. Chem. Soc. ASAP Article.
See: 10.1021/ja512903g