Ni-Catalyzed Reduction of Inert C-O Bonds: A New Strategy for Using Aryl Ethers as Easily Removable Directing Groups
Cleavage of the C-O bond in aryl alkyl ethers.
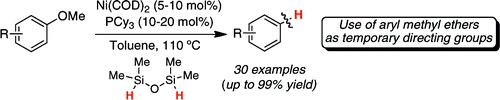
Most synthetic chemists are used to employ aryl methyl ethers as directing groups. They are powerful directors in substitution reactions, metallations and others. But once you are done, you are stick with them. They are not easy to remove, though some methods existed. Now, Martin et al. (ICIQ, Spain) have developed a catalytic method for the reduction of those ‘inert’ C-O bonds.
The method relies in the use of Ni(COD)2 (5-10 mol%), PCy3 (10-20 mol%), TMDSO as reducing agent (1 equiv) in toluene at 110 °C for 8-14 h. The results are quite good and a number of substrates are demethoxylated. The protocol leaves benzylic methyl ethers, methyl esters and others untouched. As demonstration of its applicability, the authors have prepared some substrates by ortho-metallation, removing then the OMe group to reach ‘magically’ prepared substrates, even with the troublesome 1,3-substitution pattern. I have already in mind a couple of substrates which we would like to test…
J. Am. Chem. Soc., 2010, 132 (49), pp 17352–17353. See: 10.1021/ja106943q