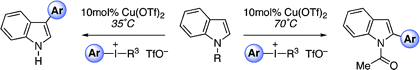Cu(II)-Catalyzed Direct and Site-Selective Arylation of Indoles under Mild Conditions
In previous issues of this newsletter, we have talked about direct C–H functionalization of indoles. Copper can make a difference.
Some C–H bonds in indoles can be selectively functionalized using organometallic catalysis. In a previous number of this issue, we have seen a recently developed method using Pd(II) to arylate the C-2 position at room temperature.
The research group of Gaunt (Cambridge, UK) has published an interesting paper to address the same problem by using electrophilic copper. Their method is based on the conversion of Cu(I) into Cu(III) by oxidation with a iodonium salt. The resulting Cu(III) complex enables a mild arylation. In a typical reaction protocol, a N-alkyl indole is arylated at the C-3 position using a [TRIP-I-Ar]OTf (TRIP being 4,6-tri-isopropylphenyl) as the oxidant and aryl source, Cu(OTf)2 (20 mol%), and 2,6-di-tert-butylpyridine (dbtpy) as the base in DCE at 35 °C. Most [TRIP-I-Ar]OTf salts can be generated in a one-step process from commercially available starting materials. The reaction gives C-3/C-2 products with high ratios (typically 20:1) and excellent yields. Interestingly, if the reaction is carried out on the N-acyl indole, the regioselectivity turns into a C-2 arylation. In this case, the C-3/C-2 ratios are not so good but yields are still excellent.
J. Am. Chem. Soc., 2008, 130 (26), 8172–8174. See: 10.1021/ja801767s