A General Palladium Catalyst System for Suzuki-Miyaura Coupling of Potassium Aryltrifluoroborates and Aryl Mesylates
More improvements on the Suzuki-Miyaura reaction partners.
The Suzuki-Miyaura cross-coupling, that is, the coupling of boronic acids to alkenyl and aryl halides has been around for many years. I remember the first years of this reaction, when boronic acids had the reputation of being hard to purify or impredictable. Today most aspects of this coupling are well stablished and using or preparing boronic acids is standard practice. A very recent improvement in the reaction is the use of trifluoroborates as coupling partners, compounds that sometimes offer advantages over the classical boron compounds.
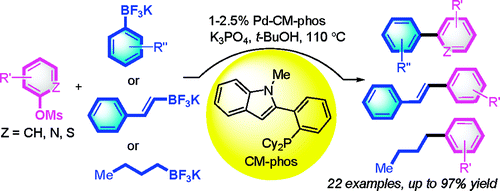
This paper by Kwong et al. (Hong Kong, China) makes a clever combination of advances in the use of mesylates as non-classical leaving groups and trifluoroborates as boronic acid surrogates. In the previous number of our issue we have presented a work by Buchwald demonstrating the coupling of mesylates with amides. Mesylates are attractive because they are more atom-economical compared to tosylates and additionally, they are less expensive and more stable than triflates. The protocol involves heating the mesylate, a trifluoroborate (200 mol%), K3PO4 (300 mol%), Pd(OAc)2 and a ligand in 1:4 ratio, in t-BuOH for 18 h at 110 °C. Results are spectacular: yields range between 65 and 97%, group selectivity is excellent, and heterocycles can be used (6 examples included).
As drawback, the key ligand is CM-phos, which unfortunately must be prepared following a published procedure. An interesting bonus is that alkyltrifluoroborates can be used also.
J. Org. Chem., 2010, 75 (15), pp 5109–5112. See: 10.1021/jo100846t