Ipso-Borylation of Aryl Ethers via Ni-Catalyzed C–OMe Cleavage
The OMe moiety used as entry point for the borylation of aryl and benzyl substrates.
Some time ago we published here one of the most striking metal catalyzed reactions developed in the last years: the breakage of the aryl-O bond in aryl methyl ethers. Striking because until now that bond was considered unbreakable, unless you do some unspeakable things or convert the OMe in a more manipulable moeity.
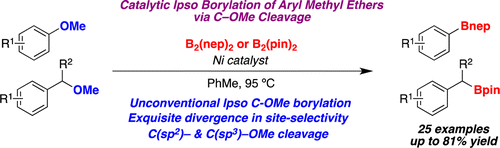
This new paper by Martin et al. (ICIQ, Spain) is a follow up of their previous work, with improvements. The use of the cocktail Ni(COD)2 (10%), PCy3 (20%) and HCO2Na (3 equiv) in toluene at 95 °C, in the presence of B2(nep)2 (sounds exotic, but it is similar to diboronpinacolate) gives the expected products with good yields. A typical byproduct of the reaction is the removal of the OMe to give the naked C-H bond. These conditions are applied to several substituted naphthalenes and benzenes, checking conditions and substrate scope.
But interestingly, the nature of the boron reagent has some influence on the outcome. If B2(pin)2 is used as boron source, a C(sp3)-OMe cleavage is observed, while using B2(nep)2 cleavages the C(sp2)-OMe bond. And when they introduce [I]+ (NaI with chloramine T), they observe an Ipso-halogenation. So in summary, they can cleavage selectively aryl methyl ethers or benzyl methyl ethers… and manipulate them to prepare boron compounds and other things. In the supporting info the authors include a brief section with substrates that fail to give the borylated compound. It seems the reaction is pretty sensitive to steric hindrance, because ortho susbtituted arenes do not yield product.
One criticism: the paper is so densely packed that reading it is difficult. Some expanded tables would enhance the readibility very much.
J. Am. Chem. Soc. 2015, Article ASAP.
See: 10.1021/jacs.5b03955