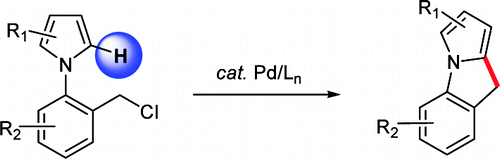Synthesis of Condensed Pyrroloindoles via Pd-catalyzed Intramolecular C–H Bond Functionalization of Pyrroles
A new method for the preparation of pyrroloindoles via Pd C–H activation.
Condensed heterocyclic compounds have important roles in medicinal chemistry and others. Many of these compounds are prepared through careful cyclization of structures with reacting groups in the appropriate positions. This involves the preparation, sometimes with great effort, of the correct building blocks: enamines, unsaturated compounds, and others.
The paper of Chang et al. (KAIST, Korea) presents an interesting approach directed to the preparation of pyrroleindoles. Their strategy is based on the use of N-(2-halobenzyl)pyrroles, with the central ring being built using the benzylic position and the C-2 position of the pyrrole by means of a Pd catalyst. This approach does not need a reactive group in the C-2 position since the Pd catalyst activates the C–H bond.
The trick of the protocol is the use of a ligand, 2-(di-tert-butylphosphino)biphenyl, in combination with Pd(OAc)2 and Et3N in benzene at 100 °C. Under those conditions, the authors report good to excellent yields (60–97%). The method tolerates carbonyl groups and chlorine atoms. I wonder whether this method can be extended to other N-(2-halobenzyl) heterocycles.
J. Am. Chem. Soc., 2008, 130 (48), pp 16158–16159. See: 10.1021/ja806897h