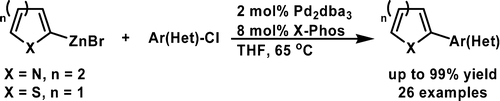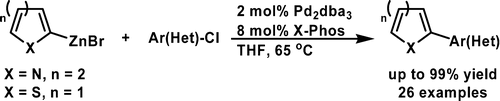A Mild Negishi Cross-Coupling of 2-Heterocyclic Organozinc Reagents and Aryl Chlorides
Improved procedure for the Negishi coupling.
We have mentioned in previous issues of our newsletter that the Negishi reaction, the coupling of aryl halides and organozinc reagents, is a powerful but underused method for the creation of C-C bonds. The reaction has some drawbacks coming from the preparation of the organozinc partners, which usually requires a methalation with a lithium reagent or the use of activated Zinc (Rieke Zinc mostly).
The chemists from Merck USA have published a short note on improved conditions for the synthesis of organozincs and the subsequent Negishi coupling specially well suited for heterocycles and intented for a scale up. First, to isopropylmagnesium chloride is added the corresponding heteroaryl halide at a temperature not exceding 30 °C; after 3-4 hours, the reaction is quenched with a solution of zinc chloride. Without isolation, this reagent can be used in the coupling, just adding it to a vessel containing the aryl halide, Pd2(dba)3 (2 mol%) and X-Phos (8 mol%) in THF. The reaction needs only about 65 °C and once completed needs no special treatment. Several examples are shown, including many with 2-pyridyl zinc chloride. Next time you think about coupling two fragments, give this reaction a try!
J. Org. Chem., 2010, 75 (23), pp 8330–8332. See: 10.1021/jo1018798