Aminocarbonylation of Aryl Tosylates to Carboxamides
Generation of aromatic amides using CO, amines and aryl tosylates as coupling substrates.
Generation of aromatic amides using CO, amines and aryl tosylates as coupling substrates.
This is one of those papers that make reading scientific literature a pleasant experience. A study started with clear goals and needs, based on a good hypothesis and a solid design of experiments. And they do NOT confuse screening with optimization.
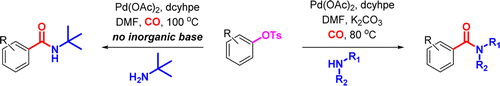
Chung and Wright (Pfizer, USA) state that they were in the need to obtain carboxamides from phenols through a palladium catalyzed carbonylation. And obvious answer is using triflates, but to reduce costs and the environmental impact of the triflate ion, they turned to tosylates, with the subsequent search for new experimental conditions. After some screening using specialized equipment they found a starting set of conditions which they modified further to get a protocol that most labs can handle: Pd(OAc)2, dcyhpe (a very electron rich phosphine from the bis(dicyclohexylphosphino)alkane family) and CO in DMF at 100 °C.
After that, the usual exploration of scope and limitations. The reaction works fine with the usual moieties (ethers, esters, carbonyles, nitriles, CF3) and interestingly no problems when there are methoxy groups in the ortho position. But the protocol works much better if you have an electron withdrawing group in the aryl tosylate, specially with some amines. Only two examples of heterocycles, unfortunately. And finally, the protocol handles aliphatic (primary and secondary) and aromatic amines.
Org. Lett. 2015, 17(11), pp 2848?2851.
See: 10.1021/acs.orglett.5b01283