Pd-Catalyzed Cross-Coupling Reactions of Amides and Aryl Mesylates
Still thinking about triflates for a coupling? Think again.
The introduction of a nitrogen atom via an organometallic coupling has been one of the most active fields in Organic Synthesis in recent years. Two names stand out: Buchwald and Hartwig. The so-called palladium catalyzed amination of aromatic compounds is therefore known as Buchwald-Hartwig reaction. Along the years, many conditions have been developed, allowing the coupling of a broad range of amines and amides with haloaromatic compounds and other suitable partners, as triflates and tosylates.
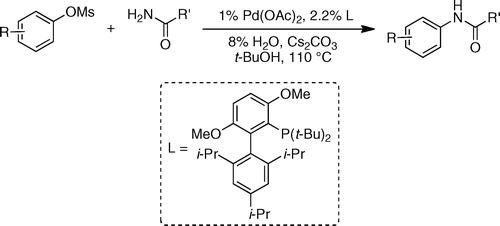
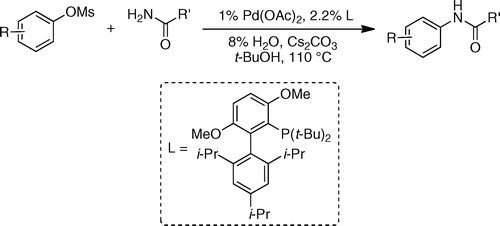
This new raid of Buchwald into the amination field extends further the range of conditions available to chemists. In this case they have centered their efforts into using mesylates as coupling partners. Mesylates are attractive because they are more atom-economical compared to tosylates and additionally, they are less expensive and more stable than triflates. The new protocol is, as usually, straightforward: The mesylate and an amide are mixed with Pd(OAc)2 (1 mol%), t-BuBrettPhos (2.2 mol%), H2O (8 mol%) and Cs2CO3 in t-BuOH at 110 °C. Depending on the substrate the reaction time ranges from 20 min to 4 h with excellent yields.
Several examples with heteroaromatic mesylates are given. The amide component can also be (hetero)aromatic or aliphatic, so the chemical space is properly explored.
Org. Lett., 2010, 12 (10), pp 2350–2353. See: 10.1021/ol100720x