Highly Regioselective Palladium-Catalyzed Direct Arylation of Oxazole at C-2 or C-5 with Aryl Bromides, Chlorides, and Triflates
Switching the coupling position in oxazoles using reaction conditions.
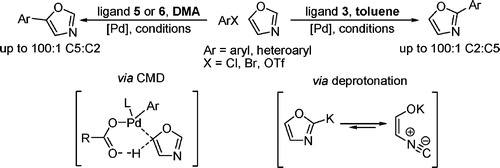
As we have commented once and again in previous issues of our newsletter, C-H activation is one of the hottest topics in Organic Synthesis. The chemists at Merck USA have just published a new paper on the direct arylation of oxazole. Oxazole is readily available and inexpensive compared to dihaloazoles, and additionally, direct arylation allows the use of many different aryl and heteroaryl halides, which are far more numerous than boronic acid coupling partners. Most importantly, direct arylation takes place at both C-2 and C-5, which is complementary to the 2,4-substitution pattern accessible through conventional Suzuki chemistry developed by the same authors.
* The most interesting part of this work is how the arylation can be switched between C-2 and C-5 just changing the ligand and solvent used: catalyst is exactly the same, the old good Pd(OAc)2. Thus, when oxazole is treated with an aryl halide, Pd(OAc)2 (10 mol%), CataCXium A or 3,4,5,6-tetramethyl-t-Bu-X-Phos as ligands (10 mol%), K2CO3 (300 mol%), pivalic acid (40 mol%) in DMA at 110 °C for 16 h, the C-5 arylation product is obtained in up to 100:1 ratio. However, if the ligand is changed to RuPhos and the solvent to toluene, the C-2 arylation product is obtained in up to 100:1 ratio.
Good news are that all ligands (even CataCXium A, what a name!) are commercially available. Yields range between 60 to 90%, with only a modest 34% for the arylation of oxazole with 2-bromo-4-fluoropyridine. It seems that starting with oxazole you can prepare now almost any substitution pattern you need.
Org. Lett., 2010, 12 (16), pp 3578–3581. See: 10.1021/ol1011778