Transition-Metal-Free Hydration of Nitriles Using Potassium tert-Butoxide under Anhydrous Conditions
A simple, controlled hydrolisis of nitriles using potassium tert-butoxide.
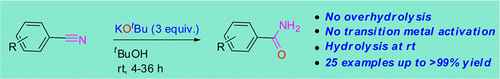
This is one of those methods that, being really simple, can make a difference in the lab. No special catalysts, hazardous reagents or cumbersome procedures. Dash et al. (Indian Association for the Cultivation of Science) have published a really nice paper describing a new protocol for the controlled hydrolisis of nitriles… in anhydrous conditions. Obviously the oxygen and protons needed for the hydrolisis do not come from water or hydroxide anions, but from potassium tert-butoxide.
The protocol is quite simple. Mix the nitrile, KOtBu and tBuOH or toluene, all anhydrous and under inert atmosphere, stir at r.t. for some hours et voilá. They have tried it with a good selection of ortho-, meta– and para-substituted benzonitriles, including a couple of heterocycles, and works also with aliphatic nitriles, but as expected with worse results. I would like to praise the authors for the experimental design, not only the selection of substrates but the experiments done to elucidate the reaction mechanism. Good job.
J. Org. Chem. 2015, ASAP Articles.
See: 10.1021/jo502752u