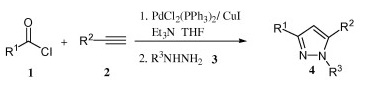One-Pot Three-Component Synthesis of Pyrazoles Through a Tandem Coupling-Cyclocondensation Sequence
A new method for the one-pot synthesis of pyrazoles from cheap, simple starting materials.
As pyridines are challenging, pyrazoles are usually rather grateful compounds. There are many methods for pyrazole synthesis, most of them involving the condensation of 1,3-diketones with hydrazines. Some one-pot syntheses of pyrazoles have been developed, but they rely on intermediates generated in situ, such as diazo compounds and ynones, that are toxic, sensitive to air, and hazardous.
The method developed by Jiang (Guangzhou, China) is aimed to solve some of these problems by using the ynones in a one-pot procedure. An suitable acyl chloride is coupled with an acetylene using PdCl2(PPh3)2, CuI and Et3N in THF at room temperature. To the resulting ynones is added a hydrazine to obtain the pyrazoles. The yields are in the 30–90% range, but so far only examples with an aliphatic alkyne have been provided. Interestingly, the method is applied also to the synthesis of a pyrimidine using acetamidine as the nitrogen source.
Tetrahedron Lett., 2008, 49 (23), pp. 3805–3809. See: 10.1016/j.tetlet.2008.03.153