The Introduction of Ammonia
Methods for the introduction of ammonia and ammonia surrogates.
The introduction of a nitrogen atom via an organometallic coupling, that is, the construction of a C–N bond, has been one of the largest growing fields in the last years. When I did my PhD, the Ullman coupling was still universally considered as ‘old and unreliable’. No palladium methods were available and a working, reliable C–N coupling was just a dream. Today, this type of coupling is available in several different (and reliable) flavors. But there still is room for improvement. One of the burning problems is the direct introduction of an NH2 moiety.
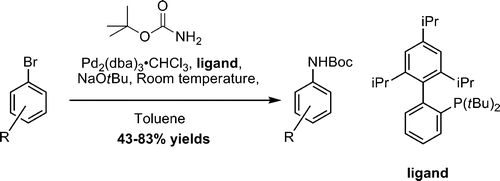
Three new papers have been recently published on this topic. The first one is a work by Hornberger et al. (GlaxoSmithKline, USA). The origin of their work is directly connected to the need for access a number of arylamines with a suitable protecting group. The protected ammonia source is tert-butyl carbamate, since the Boc group is convenient and robust. But no systematic work had been done on the use of this source with the last coupling conditions. The new protocol uses Pd2(dba)3·CHCl3, tert-butyl X-Phos as the ligand, and NaOtBu as the base in toluene. The most outstanding point is that the coupling is carried out at room temperature. In other words, no heating, no microwaves. Yields are usually excellent and over 70%. 3-Bromopyridine is included as an example, with a 47% yield reported.
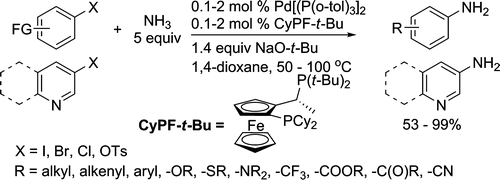
The third paper is the icing on the cake and will probably replace the precedent methods in the following years. Hartwig and his collaborators (Urbana, IL, USA) have attacked the same problem of the direct coupling with ammonia, but using palladium instead copper. The reaction protocol consists in a complex generated from Pd[P(o-tol)3]2, CyPF-t-Bu (Josiphos), using NaO-t-Bu as the base in 1,4-dioxane between 80 and 100 °C. This method can be applied to aryl chlorides, bromides, iodides and sulfonates with a very wide range of substituents, giving the corresponding aryl amines in excellent yield. Some examples with nitrogen heterocycles are included, and even a comparison with other copper-catalyzed ammonia-coupling protocols.
Room-Temperature Pd-Catalyzed Amidation of Aryl Bromides Using tert-Butyl Carbamate
J. Org. Chem. 2009, 74 (12), pp 4634–4637. See: 10.1021/jo9004537
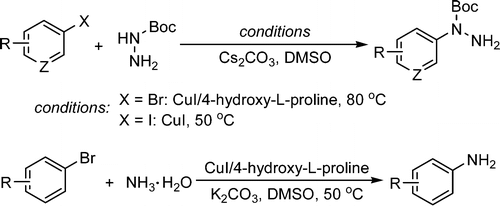
CuI/4-Hydro-L-proline as a More Effective Catalytic System for Coupling of Aryl Bromides with N-Boc Hydrazine and Aqueous Ammonia
J. Org. Chem. 2009, 74 (12), pp 4542–4546. See: 10.1021/jo9006738
Palladium-Catalyzed Coupling of Ammonia with Aryl Chlorides, Bromides, Iodides, and Sulfonates: A General Method for the Preparation of Primary Arylamines
J. Am. Chem. Soc. 2009, 131 (31), pp 11049–11061. See: 10.1021/ja903049z