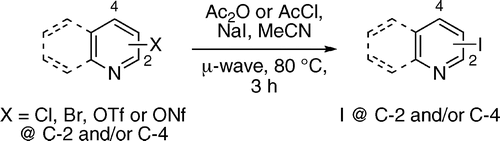Microwave-Assisted trans-Halogenation Reactions of Various Chloro-, Bromo-, Trifluoromethanesulfonyloxy- and Nonafluorobutanesulfonyloxy-Substituted Quinolines, Isoquinolines, and Pyridines Leading to the Corresponding Iodinated Heterocycles
A method for the introduction of iodine into functionalized nitrogen heterocycles.
First fact: aryl iodides are excellent substrates for organometallic couplings. Second fact: aryl iodides are often difficult to obtain, specially if we are talking about heteroaryl iodides. Conclusion: a method to obtain efficiently iodinated heteroaromatic compounds is a good thing. Banwell (Canberra, Australia) is well known for his extensive work on nitrogen heterocycles and, in this paper, we can find a solution for one of those ‘minor’ and irritating challenges. Formally, the method is an SNAr reaction using iodide as the nucleophile and an acylated heterocycle as the electrophile, with chloride, bromide, triflate or nonaflate as the leaving group.
As an example, a solution of the nitrogen heterocycle, sodium iodide, and acetic anhydride in acetonitrile is heated at 80 °C under microwave irradiation. Although the temperature is relatively low, ensuring survival of sensitive substrates in the reaction media, the time required is quite long: 3 h. Alternatively, acetyl chloride can be used, especially when isoquinoline and pyridines are the substrates. However, the yields obtained are excellent, usually higher than 90%, and the method can be applied to quinolines, isoquinolines and pyridines.
J. Org. Chem., 2009, 74 (13), pp 4893–4895. See: 10.1021/jo9008386