Ruthenium-Catalyzed Cycloaddition of Aryl Azides and Alkynes
The use of a Ru catalyst allows now the preparation of 1,5-disubstituted triazoles under microwave irradiation. Another useful tool for medicinal chemists.
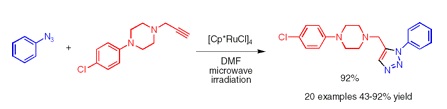
The importance of triazoles is well known. One of the most popular synthetic routes toward these compounds is the copper(I)-catalyzed azide-alkyne cycloaddition, which yields 1,4-disubstituted triazoles. Recently, a similar ruthenium-catalyzed cycloaddition has been shown to provide access to 1,5-disubstituted compounds. However, this reaction suffers from low yields and formation of byproducts. Now, the research group of Fokin (Scripps, CA, USA) has published a short paper reporting an improved, microwave-driven method to carry out the cycloaddition. Besides microwaves, the key to such efficient cycloaddition is the use of [Cp*RuCl]4, which can be easily prepared from commercial RuCl3·H2O.
Org. Lett., 2007, 9 (26), pp. 5337–5339. See: 10.1021/ol701912s