A Powerful Reagent for Synthesis of Weinreb Amides Directly from Carboxylic Acids
A new reagent for the formation of Weinreb’s amides from carboxylic acids in one step.
Weinreb amides (N-methoxy-N-methylamides) have many important synthetic uses. Their reactivity can be summarized as that of a controlled-reactivity acylating agent. They react with Grignard and organolithium reagents to give ketones, instead of tertiary alcohols as esters do. If LAH is used, they give aldehydes and not alcohols and, very recently, it has been reported that you can have a ketone also by reaction with a Wittig ylide. However, the reaction conditions for the preparation of these amides from carboxylic acids are still far from perfect. The use of a big excess of the coupling reagent is needed, and other methods are not much better.
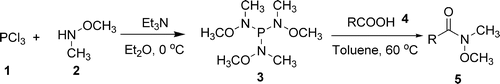
Huang (Lanzhou, China) and Hu (Guanxi, China) have now published the results of a combined effort to develop a new, more effective method. They have discovered that P[NCH3(OCH3)]3 can be prepared and used to carry out the desired transformation in a very efficient manner. The protocol involves heating the carboxylic acid and the reagent (2:1 molar ratio) in toluene at 60 °C for 30–60 min. The Weinreb amides are obtained in excellent yields, usually higher than 90%. Examples with both aromatic and alyphatic acids are published, including a thiophene and a furane.
Org. Lett., 2009, 11 (19), pp 4474–4477. See: 10.1021/ol901886u