Selective Copper-Promoted Cross-Coupling of Aromatic Amines with Alkylboronic Acids
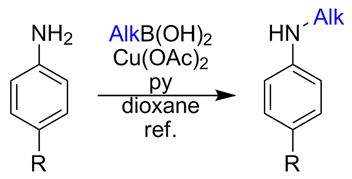
In 2009, we published our results on the selective monomethylation of anilines and other heterocyclic amines through a copper-promoted cross-coupling with methylboronic acid. We would like to present now an expansion of the work that allows the selective introduction of many different alkyl groups into aromatic amines, a method that many medicinal chemists will welcome as a very useful addition to their toolkit.
Although selective monoarylation of anilines and other aromatic amines can be easily achieved, monoalkylation is still an underdeveloped reaction. Direct alkylation is typically accomplished by reaction with an alkyl halide or similar reagent in the presence of a base. It seems to be a simple transformation but, in many cases, efficient N-monoalkylation of primary anilines is not possible due to over alkylation, which affords tertiary anilines or quaternary ammonium salts as by-products. Other methods for the preparation of monoalkylated anilines are based on the use of a temporary protecting group (e.g., carbamate, benzyl) that allows the introduction of a single alkyl group, followed by removal of the protecting group (introducing two additional steps in the process). Reductive amination with carbonyl derivatives and amide reduction are also used as alternative approaches for the preparation of secondary amines; these are selective methods, but the reaction conditions are somewhat harsh, as strong reducing reagents must be used.
Following our efforts on the monomethylation of anilines using the Chan–Lam reaction, we decided to expand the work to other alkyl groups. We found that the reaction conditions developed for the methylation can be used with a broad range of alkylboronic acids, including propyl, isopropyl, butyl, isobutyl, cyclohexyl, cyclohexylethyl, phenylethyl, among others. The reaction works with good yields over anilines bearing electron donating and withdrawing groups, with no dialkylation products being detected.
We were especially interested in the synthesis of phenethylamine derivatives because of the range of active compounds that present this structure. Despite the importance of these derivatives, there are few methods to incorporate phenethyl groups on nitrogen atoms. During our nine years in the medicinal chemistry business we had our share of problems introducing this group into very different scaffolds, so a more effective method would be a nice addition to our toolkit.
An optimized procedure for the reaction with the phenethylboronic acid was developed. The reaction can be run under mild reaction conditions and good functional group tolerance. Carbonyl, nitrile, halide, thioether, and other groups can be present in the molecule, with yields ranging from 60% to 90%. Even more interesting, unlike the previously protocol of monomethylation, the reaction works also on aminopyridines, which opens many interesting venues.
This work has been submitted for publication in Synlett, and a further extension of this new reaction is currently under investigation in our labs.
Updated on January 23rd, 2015
Selective Copper-Promoted Cross-Coupling of Aromatic Amines with Alkyl Boronic Acids
Synlett 2010 (14), pp 2101–2105.
See: 10.1055/s-0030-1258523