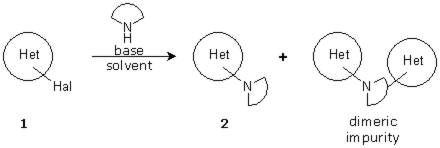
One of our clients approached us with a request to optimize the preparation of an heterocyclic scaffold used as starting point for a drug discovery route. The product was obtained through the SNAr reaction of a chloroheterocycle with a high-value amine. Although the reaction worked, it was slow, conversion was low, and a dimeric impurity was present in significant quantities. These problems translated into low yields (34% after 3 days) and difficult purification procedures.
Time was of paramount importance. A fast bibliographic search showed that similar reactions had been described using metal couplings, but not over our scaffold, so to avoid longer development times, metals were discarded. Although an obvious improvement was the application of microwave conditions, this alternative was also discarded to avoid scaling-up issues. We concentrated our efforts in finding a new combination of solvent and base, so we designed a fast screening evaluating 9 different solvents and 2 bases, using as response factors the percentages of starting material and product obtained by LC-MS. Not the 18 possible combinations were considered, since some hints available in the bibliography led us to expect bad results from some combinations. An initial set of six reactions was carried out in parallel in a Radley’s carousel.
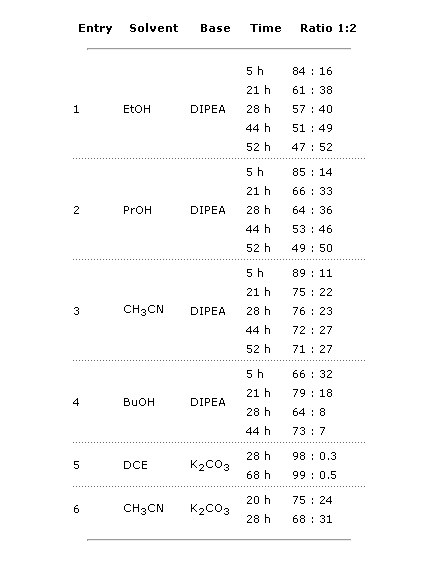
Initially, all reactions were carried out at 86 °C with 1.5 equiv of base. The reactions were monitored at different times, and soon it was clear that more base was needed, so 2 additional equiv of the corresponding base were added to each reaction. Some conclusions were drawn:
- EtOH and PrOH with DIPEA (entries 1 and 2) gave similar results, with 50% conversion after 52 h. No dimeric product was detected.
- CH3CN with DIPEA (entry 3) furnished a mediocre result, with 27% conversion after 52 h, but traces of dimeric product were detected.
- BuOH (entry 4) afforded the worst results, with increasing quantities of the dimeric compound being detected in each sample.
- DCE with K2CO3 (entry 5) was definitely a no-go, with no conversion.
- CH3CN with K2CO3 (entry 6) gave also acceptable results. Although the conversion was lower than using DIPEA, no dimeric product was detected.
Results with EtOH and PrOH were promising. CH3CN showed also potential, with good conversion at shorter reaction times. The next batch of experiments was set up to include other polar solvents with 3.5 equiv of base from the beginning. Additionally, since conversion was quite similar at 44 and 52 h, a maximum time was established at 44 h.
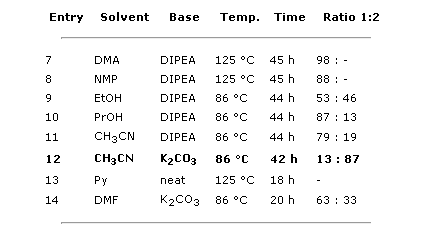
The results from the second set of experiments were much better:
- Entries 7, 8, and 14 show that other polar solvents do not improve the CH3CN result.
- Entries 9 and 10 reflect that a full quantity of base from the beginning gives no improvement with alcohols.
- Entry 11 reflects a similar behavior for CH3CN with DIPEA: no improvement.
- Entry 12 shows that, for CH3CN with K2CO3, a full quantity of base from the beginning makes a difference.
- Entry 13 shows that pyridine, which is at the same time the solvent and base, gives no product.
Clearly the entry 12, 3.5 equiv of K2CO3 in CH3CN for 42 h at 86 °C is the winner. But in fact, the reaction time can be shortened. A sample of the reaction monitored by LC-MS at 24 h showed a 16:83 ratio, not much worse than the final 13:87. So we decided that a 1–2% increase in conversion was not worth 24 additional hours. The reaction was finally reproduced at a larger scale with similar results: 12:87 ratio at 26 h.
Those were the conditions given to the client in a full experimental report including the results of each run. Note that in fact the reaction is not complete after 26 h, but no dimeric product is detected and purification is much easier than with the previous conditions.