In the next months, we will publish a couple of papers on our research on organometallic couplings. Even if they are not accepted, these papers are clearly in the field of methodology, as opposed to those papers aimed at the synthesis of a single target. There are clear differences between both types of papers and this time we would like to share with you our views on those works developing new methodologies.
I would like to start saying that this editorial reflects my personal ideas and not those of GalChimia. We could write down a disclaimer here, but I don’t think it is necessary. Maybe some of the comments you are going to read (well, if you don’t quit reading right now) will stir some unease, but these exercises of thought must be done.
As I commented in the introduction, methodology papers are a different class. In a total synthesis paper, the aim is to prepare the target, and once the strategy is outlined, you can use the tactics you consider more convenient. For example, if your strategy relies on the coupling of two fragments, you can choose among many options to carry out the coupling. The question is not the type of coupling you do, but doing a coupling. Sometimes you will see papers where some restrictions are applied: that type of reaction has been done before in a strategy like this, and now we want to apply this other reaction. That enters into a subcategory ‘And now, even more difficult!’.
But methodology papers have another aim. Usually you look for a new specific transformation, a new way of doing things that overcomes the problems of current methods, or a modification of known conditions to new substrates. You can think easily on dozens of papers fitting each subcategory. To summarize, you are looking for different ways of doing things. What counts here is not so much the place where you go, but the way you create. If you think about it, what we try to do with our newsletter is to provide the medicinal chemists out there with useful methodology papers.
I must confess that I love methodology papers. In our line of work, I usually find them much more useful than those describing total synthesis, because what our clients request are new things, so you read the papers looking for conditions that could be applied to your problem. The usual question is, ‘ok, will those conditions work with my substrate?’ That’s why sometimes is so annoying reading some methodology papers. They describe a wonderful new reaction that you can use for ongoing or future projects but… you see some gaps.
Those gaps are usually related to the most formal aspect of DoE. Simply, a failure of design. For example, you are using substituted iodobenzenes as substrates and you see examples where substituents are 4-chloro and 4-fluoro, but the 4-bromo is missing. Or you see the 4-methoxy and 3-methoxy, but not the 2-methoxy. Sometimes it seems somebody selected the substrates by saying, ‘ok, what substrates of this type I have on the storage room?’; but, what happens with the missing substrate? Thinking the worst of people, the substrate is missing because the result was awful and they did not want to spoil the results. So you do not have critical information about how the reaction works with that substrate and similar ones. Or thinking the best of people, no serious effort was put into the design, in terms of what information they could obtain from the experiment. And sometimes it is very, very frustrating. So if you have reached this point, I will share with you what I would like to see in a proper methodology paper and, in fact, what we try to do when we do research and publish a methodology paper.
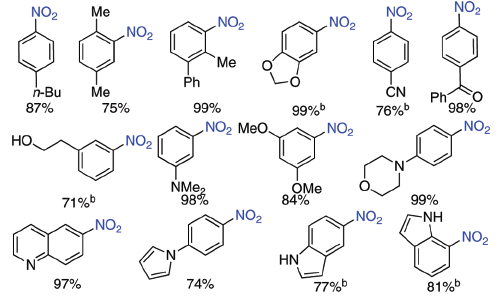
The above figure is extracted from a paper where the introduction of a nitro group on aryl iodides is described. Thus, palladium coupling allows the substitution of an iodine atom by a nitro moiety (in blue). Electron-rich and electron-deficient substituents are present in the compounds of the first two rows. However, no effort was made toward exploring complete series of compounds, and some info on selectivity would be welcomed. Some easy questions that come to mind:
- What would happen if a methoxy group or another coordinating moiety is present at the ortho position?
- If you have another halogen in the starting material, it remains unaffected or will you have selectivity problems?
- Pyridines are important substrates and they are hard to nitrate. Why haven’t pyridines been included?
- Polynitration is an issue. Why not using an halonitrobenzene as the substrate?
Honesty, above all, if a substrate does not work or the result is bad, include it anyway. You are being honest and helping other chemists by telling them not only what works, but what does not work. Second, try to build your tables thinking in terms of DoE. Think why you are including that substrate in the table, not only because ‘hey, I have it on the shelf!’, but because the information you obtain from the experiment is valuable. For example, if you include the methoxy substituent as an example of an electron-rich substituent, contemplate how it affects the reactivity in all the different positions. Think on electron-rich, neutral, and electron-deficient substituents. Think how sulfur substituents can poison a catalyst or whether other halides can also react. Include the substituents you want to demonstrate are compatible with the protocol. And finally, comment your results! People reading the paper maybe have not a clue about why you selected that substrate or what you try to demonstrate with that particular experiment. Reading something like ‘entries 5–10 demonstrate that the reaction protocol is compatible with moieties sensitive to reducing agents’ helps a lot and highlights the advantages of your protocol, both for the readers and the referees.
